Goal: 600-700 horsepower on 87 Octane pump gasoline from a stock, junk yard special, LS series small block
4.8l LR4 (LSx series, Vortec 4800 truck engine)
Displacement 4807cc (293 ci)
Bore and Stroke (in.) 3.78 x 3.26
Compression Ratio 9.5:1
Recommended Fuel regular unleaded
Valve Lifters hydraulic roller
Horsepower 285 hp ( 213 kw ) @ 5200 rpm
Torque (lb. ft. @ rpm) 295 lb-ft ( 400 Nm ) @ 4000 rpm
Maximum engine speed 6000 rpm
Cylinder block material cast iron
Cylinder head material cast aluminum
3 turbos, 2 feeding one, compounded in series, each capable of (32.5 - 40) 35lb/min @ a pressure ratio of 3 for a total pressure ratio of 9 and max air flow of 70lb/min
70°F Ambient Temp
70°f Water Temp
14.7 Ambient Pressure
0 humidity
[HR][/HR]
Alright, groundwork laid out. I spent a little time figuring all this out. It's conceptual, so input and corrections are welcome. Here goes...
Two turbos produce 70lb/min @ a pressure ratio of 3 and is fed into a 3rd torbo, also with a pressure ratio of 3.
3 x 3 = a total pressure ratio of 9
9 x 14.7 (ambient pressure) = an absolute pressure of 132.3 psia. Subtract 14.7 to get pressure over ambient, or a gauge pressure of 117.6psig.
Not counting any heat added by the turbos, but strictly looking at the heat of compression, we can take the intake (ambient) temperature of 70°F and (using google) convert it to kelvin, or absolute temperature. A measure of total heat energy above absolutely none at all...
70°F = 294 kelvin
Using the following equation we can find the compressed heat:
![Image]()
Where:
T1 = 70°F = 294.261kelvin
P1 = 14.7psi or 1 bar
P2 = 132.3 psia or 9 bar absolute pressure (must use absolute pressure, not gauge pressure)
k = 1.4 (the Heat Capacity Ratio for dry air)
So, solving the exponent (k) first, 1.4 -1 = 0.4 / 1.4 = 0.286
Then the pressure ratio (P2/P1) which we already know from above, 132.3 / 14.7 = 9
Pressure ratio of 9 to the power of 0.286 = 1.875
1.875 x 294.26 kelvin = 551.74 kelvin, or (using google) 533.46°F
I didn't account for heat added by the compressor because in theory it's going to get removed anyway. It would be good to know in calculating the required BTU capacity of the intercooler/aftercooler, I just haven't got that far yet. Calculating the heat increase isn't all to important at this point, but the formula will be used again in just a moment to calculate the temperature upon decompression...
[HR][/HR]
At this point we have hot compressed air, we can use the information thus far to calculate it's new volume, or volume ratio, or compression ratio (Not the engine's compression ratio) using the following equation...
CR = T1 / T2 x PR
If:
T1 = 294k
T2 = 552k
PR = 9
294 / 552 x 9 = 4.79:1 Compression Ratio
So if the volume of the inter/aftercooler and related piping was 10 cubic feet there is now 47.85 cubic feet of air in there. Which isn't actually all true, we can stuff more in there as it cools.
You can also look at it as 1 / 4.79 = 0.209, or what was one cubic foot is now 0.209 cubic foot. Or 100 cubic feet is now 20.9 cubic feet. Just another mental viewpoint.
[HR][/HR]
So, what if we were able to remove all of this extra heat with an a/w intercooler, or even... say, 4 a/w intercoolers in series, returning the temperature back to 70°F? What would the compressed volume of what was one cubic foot be, if it's still at 132.3psia?
![Image]()
Using the first equation above and the new volume of one cubic foot of 0.209...
V1 = 0.209 cubic foot
T1 = 552k
T2 = 294k
Then, 0.209 / 552 x 294 = 0.111 cubic feet after cooling back to ambient.
1 / 0.111 = 9
In a space of ten cubic feet there is there is now 90 cubic feet of air
So, the new compression ratio, assuming a constant pressure ratio of 9 is now 9:1. Which I found interesting. If you are going to remove all the heat in your calculations anyway, you can skip a lot of the mumbo jumbo math stuff above and just use your/the total absolute pressure ratio we started with in the beginning, no matter what it is..
[HR][/HR]
Alright, so here's where it get's interesting. If, after the last intercooler (of 4 mentioned above), there is a pressure regulator that regulates boost into the throttle body and intake manifold, you could set the manifold pressure at any psi you like even though there is 117.6psig (132.3psia) in the intercooler.
If the engine makes 300hp and we want 600, it's commonly understood we need to double atmospheric pressure. So, set the regulator for 14.7psig (29.4psia).
Now, I want to find out what the temperature of the air is going to be once it's decompressed from 70°F @ 132.3psia to 29.4psia.
Using the same equation from above:
![Image]()
k = 1.4 so the exponent is 0.286
P2 = 29.4 absolute (14.7 psig)
P1 = 132.3
T1 = 294
So, 29.4 / 132.3 to the power of 0.286 = 0.6504
0.6504 x 294 = 191 kelvin = -115.87°F YES. NEGATIVE 115.87°F
Dry ice is -109.3°F. So, the intake charge would be colder than dry ice.
[HR][/HR]
So here's where it get's doubly interesting.
If you were to NOT create boost and simply cool ambient pressure air from 70°F to -116°F, it would have a supercharging effect on it's own, because one cubic foot of air at 70°F is 0.65 cubic foot @ -116°F. So as the engine draws in one cubic foot it's getting the equivalent of over 1.5 cubic feet. (1 / 0.65 = 1.54) So doubling that with pressure is 3 times the amount of air into the cylinder. If my math is correct ( 29.4psia x 0.65 =) 19.11psia now needed to make the hp double. 19.11 - 14.7 = a boost pressure of only 4.41psig :shock:
Volume change found, again, with the first equation below
![Image]()
V1 = 1 cubic foot
T1 = 294k
T2 = 191k
Then, 1 / 294 x 191 = 0.65 cubic feet
[HR][/HR]
Alright! So, can 600-700hp be made with 87 octane pump gas without detonation?
In searching the internet I've found that a stock LS1 is capable of 99.7% volumetric efficiency at max torque. I don't know if it's true and the 4.8 isn't an LS1, but that's the number I'll use for my calculations.
The (calculated) compression ratio of the 4.8 LR4 is 9.5:1.
99.7% of 9.5 = 9.4715
To convert this from a compression ratio to a pressure ratio, simply take the compression ratio to the power of 1.4, the Heat Capacity Ratio for dry air.
9.4715 to the power of 1.4 = 23.28 pressure ratio
So the highest pressure in the cylinder (before ignition) should be 23.28 x 14.7psia = 342.22psia (328psig)
Using these numbers we can calculate the temperature the air reaches on it's compression stroke.
![Image]()
T1 = 70°F = 294k
P1 = 14.7psia
P2 = 342.22 psia
k = 1.4 -1 = 0.4 / 1.4 = 0.286
342.22 / 14.7 = 23.28
Pressure ratio of 23.28 to the power of 0.286 = 2.46
2.46 x 294kelvin = 723 kelvin = 842°F at the top of the compression stroke
Now, if we run 14.7 pounds of boost @ -116°F...
29.4psia x 23.28 pressure ratio = 684psia
684 / 29.4 = 23.28 (redundant, but shows the relation)
23.28 to the power of 0.286 = 2.46
2.46 x 191k = 470k = 386°F at the top of the compression stroke
(It's worth noting that the above calculation would actually be closer to 900hp, beyond the limits of the turbos I used for reference)
I'd say, unless I duffed on some of the math, you could absolutely run regular 87 gas.
Who needs race gas or E85???
Serious turbo guys (Mark) should be able to run the cheap stuff and gain miles per tank...
Just a theory at this point, but... Any thoughts?
4.8l LR4 (LSx series, Vortec 4800 truck engine)
Displacement 4807cc (293 ci)
Bore and Stroke (in.) 3.78 x 3.26
Compression Ratio 9.5:1
Recommended Fuel regular unleaded
Valve Lifters hydraulic roller
Horsepower 285 hp ( 213 kw ) @ 5200 rpm
Torque (lb. ft. @ rpm) 295 lb-ft ( 400 Nm ) @ 4000 rpm
Maximum engine speed 6000 rpm
Cylinder block material cast iron
Cylinder head material cast aluminum
3 turbos, 2 feeding one, compounded in series, each capable of (32.5 - 40) 35lb/min @ a pressure ratio of 3 for a total pressure ratio of 9 and max air flow of 70lb/min
70°F Ambient Temp
70°f Water Temp
14.7 Ambient Pressure
0 humidity
[HR][/HR]
Alright, groundwork laid out. I spent a little time figuring all this out. It's conceptual, so input and corrections are welcome. Here goes...
Two turbos produce 70lb/min @ a pressure ratio of 3 and is fed into a 3rd torbo, also with a pressure ratio of 3.
3 x 3 = a total pressure ratio of 9
9 x 14.7 (ambient pressure) = an absolute pressure of 132.3 psia. Subtract 14.7 to get pressure over ambient, or a gauge pressure of 117.6psig.
Not counting any heat added by the turbos, but strictly looking at the heat of compression, we can take the intake (ambient) temperature of 70°F and (using google) convert it to kelvin, or absolute temperature. A measure of total heat energy above absolutely none at all...
70°F = 294 kelvin
Using the following equation we can find the compressed heat:

Where:
T1 = 70°F = 294.261kelvin
P1 = 14.7psi or 1 bar
P2 = 132.3 psia or 9 bar absolute pressure (must use absolute pressure, not gauge pressure)
k = 1.4 (the Heat Capacity Ratio for dry air)
So, solving the exponent (k) first, 1.4 -1 = 0.4 / 1.4 = 0.286
Then the pressure ratio (P2/P1) which we already know from above, 132.3 / 14.7 = 9
Pressure ratio of 9 to the power of 0.286 = 1.875
1.875 x 294.26 kelvin = 551.74 kelvin, or (using google) 533.46°F
I didn't account for heat added by the compressor because in theory it's going to get removed anyway. It would be good to know in calculating the required BTU capacity of the intercooler/aftercooler, I just haven't got that far yet. Calculating the heat increase isn't all to important at this point, but the formula will be used again in just a moment to calculate the temperature upon decompression...
[HR][/HR]
At this point we have hot compressed air, we can use the information thus far to calculate it's new volume, or volume ratio, or compression ratio (Not the engine's compression ratio) using the following equation...
CR = T1 / T2 x PR
If:
T1 = 294k
T2 = 552k
PR = 9
294 / 552 x 9 = 4.79:1 Compression Ratio
So if the volume of the inter/aftercooler and related piping was 10 cubic feet there is now 47.85 cubic feet of air in there. Which isn't actually all true, we can stuff more in there as it cools.
You can also look at it as 1 / 4.79 = 0.209, or what was one cubic foot is now 0.209 cubic foot. Or 100 cubic feet is now 20.9 cubic feet. Just another mental viewpoint.
[HR][/HR]
So, what if we were able to remove all of this extra heat with an a/w intercooler, or even... say, 4 a/w intercoolers in series, returning the temperature back to 70°F? What would the compressed volume of what was one cubic foot be, if it's still at 132.3psia?
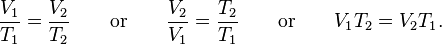
Using the first equation above and the new volume of one cubic foot of 0.209...
V1 = 0.209 cubic foot
T1 = 552k
T2 = 294k
Then, 0.209 / 552 x 294 = 0.111 cubic feet after cooling back to ambient.
1 / 0.111 = 9
In a space of ten cubic feet there is there is now 90 cubic feet of air
So, the new compression ratio, assuming a constant pressure ratio of 9 is now 9:1. Which I found interesting. If you are going to remove all the heat in your calculations anyway, you can skip a lot of the mumbo jumbo math stuff above and just use your/the total absolute pressure ratio we started with in the beginning, no matter what it is..
[HR][/HR]
Alright, so here's where it get's interesting. If, after the last intercooler (of 4 mentioned above), there is a pressure regulator that regulates boost into the throttle body and intake manifold, you could set the manifold pressure at any psi you like even though there is 117.6psig (132.3psia) in the intercooler.
If the engine makes 300hp and we want 600, it's commonly understood we need to double atmospheric pressure. So, set the regulator for 14.7psig (29.4psia).
Now, I want to find out what the temperature of the air is going to be once it's decompressed from 70°F @ 132.3psia to 29.4psia.
Using the same equation from above:

k = 1.4 so the exponent is 0.286
P2 = 29.4 absolute (14.7 psig)
P1 = 132.3
T1 = 294
So, 29.4 / 132.3 to the power of 0.286 = 0.6504
0.6504 x 294 = 191 kelvin = -115.87°F YES. NEGATIVE 115.87°F
Dry ice is -109.3°F. So, the intake charge would be colder than dry ice.
[HR][/HR]
So here's where it get's doubly interesting.
If you were to NOT create boost and simply cool ambient pressure air from 70°F to -116°F, it would have a supercharging effect on it's own, because one cubic foot of air at 70°F is 0.65 cubic foot @ -116°F. So as the engine draws in one cubic foot it's getting the equivalent of over 1.5 cubic feet. (1 / 0.65 = 1.54) So doubling that with pressure is 3 times the amount of air into the cylinder. If my math is correct ( 29.4psia x 0.65 =) 19.11psia now needed to make the hp double. 19.11 - 14.7 = a boost pressure of only 4.41psig :shock:
Volume change found, again, with the first equation below
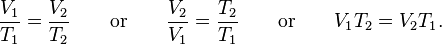
V1 = 1 cubic foot
T1 = 294k
T2 = 191k
Then, 1 / 294 x 191 = 0.65 cubic feet
[HR][/HR]
Alright! So, can 600-700hp be made with 87 octane pump gas without detonation?
In searching the internet I've found that a stock LS1 is capable of 99.7% volumetric efficiency at max torque. I don't know if it's true and the 4.8 isn't an LS1, but that's the number I'll use for my calculations.
The (calculated) compression ratio of the 4.8 LR4 is 9.5:1.
99.7% of 9.5 = 9.4715
To convert this from a compression ratio to a pressure ratio, simply take the compression ratio to the power of 1.4, the Heat Capacity Ratio for dry air.
9.4715 to the power of 1.4 = 23.28 pressure ratio
So the highest pressure in the cylinder (before ignition) should be 23.28 x 14.7psia = 342.22psia (328psig)
Using these numbers we can calculate the temperature the air reaches on it's compression stroke.

T1 = 70°F = 294k
P1 = 14.7psia
P2 = 342.22 psia
k = 1.4 -1 = 0.4 / 1.4 = 0.286
342.22 / 14.7 = 23.28
Pressure ratio of 23.28 to the power of 0.286 = 2.46
2.46 x 294kelvin = 723 kelvin = 842°F at the top of the compression stroke
Now, if we run 14.7 pounds of boost @ -116°F...
29.4psia x 23.28 pressure ratio = 684psia
684 / 29.4 = 23.28 (redundant, but shows the relation)
23.28 to the power of 0.286 = 2.46
2.46 x 191k = 470k = 386°F at the top of the compression stroke
(It's worth noting that the above calculation would actually be closer to 900hp, beyond the limits of the turbos I used for reference)
I'd say, unless I duffed on some of the math, you could absolutely run regular 87 gas.
Who needs race gas or E85???
Serious turbo guys (Mark) should be able to run the cheap stuff and gain miles per tank...
Just a theory at this point, but... Any thoughts?




